Abstract
Introduction
Development of assays for evaluating leukaemia cells' sensitivity to antileukaemia agents is emerging to be an important aspect of personalized medicine. The conventional cell cultures lack the three-dimensional (3D) structure of the bone marrow (BM), the extracellular matrix and stromal components which are crucial for the growth and survival of leukaemia stem cells. To accurately predict the sensitivity of the leukaemia cells in an in vitro assay a culturing system containing the essential components of bone marrow is required. Various 3D cultures have been developed so far for studying leukaemia cells (Aljitawi et al . 2014). We hypothesize that a scaffold containing the spongious structure of the BM by providing the niche like spaces recapitulates the resistance to targeted therapy observed in a subset of leukaemia cells in vivo .
Materials & Methods
A V-junction microfluidic device was used for bubble preparation. Calcium alginate foam-based scaffold were produced using sodium alginate solution (Ahmad et al. 2011) followed by adding Hydroxyapatite. Alginate microbubbles were collected in calcium chloride to cross-link the bubbles' shell. K562, AR230 and HL60 cell lines were cultured in RPMI medium supplemented with FBS, L-glutamine and penicillin/streptomycin. Cell proliferation and viability was measured by MTS assay. Primary normal CD34+ and acute myeloid leukaemia cells were cultured in StemSpan medium supplemented with CC100. Immunophenotype was determined using 8-color Euroflow AML/MDS antibody panel (CD16 FITC, CD13 PE, CD34 PerCP-Cy5.5, CD117 PE-Cy7, CD11b APC, CD10 APC-H7, HLA-DR V450, CD45 V500) for investigation of myeloid differentiation in normal CD34+ cells and myeloblasts from two AML patients. Data was acquired in a FACSCanto II flow cytometer (BD) and analysed with Infinicyt software (Cytognos).
Results and conclusion
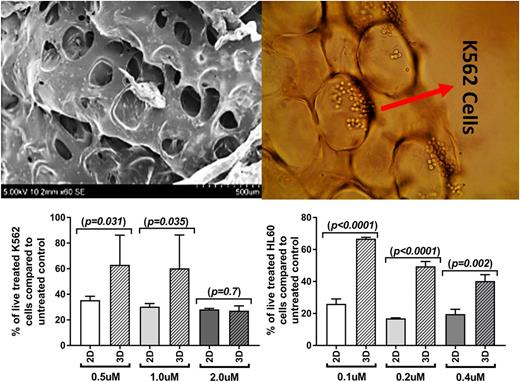
K562 and HL60 leukemia cell lines were cultured in the porous alginate foam-based scaffold (Scanning Electron Microscopy image, top left figure) followed by evaluating their sensitivity to antileukaemia agents through measuring cell proliferation. Similar to BM niches, micro-cavities in the scaffold accommodated leukaemia cells (top right figure). Foam-based scaffold reduced the sensitivity of K562 and HL60 cells to inhibitory effect of imatinib and doxorubicin respectively compared to 2D culture (low left and right figures). Combination of foam-based scaffold with BM-derived stromal HS-5 cell line further reduced the leukaemia cells' sensitivity to antileukaemia agents. Foam-based 3D culture increased the differentiation of the haematopoietic cells compared to 2D culture as normal CD34+ precursors in the scaffold showed stronger and more homogenous expression of the myeloid associated antigens CD117 and CD13. Similarly, myeloblasts from AML patients in foam-based 3D culture showed a profound monocytic differentiation compared to 2D culture after 3 days. Similar to cell lines, primary CML CD34+ and primary AML blast cells showed increased resistance to imatinib and doxorubicin respectively in foam-based 3D compared to 2D culture (Figure). The conventional 2D culture is not a highly reliable system for drug sensitivity investigations as the in vitro drug sensitivity of leukaemia cells in 2D culture is different from what is observed in vivo (Edmondson et al . 2014). Our foam-based 3D culture reduced the leukaemia cells' sensitivity to antileukaemia agents and further induced the differentiation of the normal and leukaemia cells compared to 2D culture. These differences might be attributed to microcavities mimicking bone marrow niches in foam-based scaffold. We propose to apply this foam-based 3D culture for drug sensitivity investigations and therapeutic target discoveries.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal